We educate and inform policymakers, the media and the public about the importance of doctor-patient decision-making and respond to dangerous, abusive or harmful medical device marketing practices that threaten the quality of care and jeopardize patients increased risk for harm.
Truinject champions the core of every healthcare decision, advocating for both the doctor-pharmaceutical relationship and the doctor-patient relationship because we believe in delivering quality care and ensuring patient safety outcomes.
Before you receive an injection, do your research, and if you experience a complication, report it.
Consumers should report criminal activity to the FDA at 800-551-3989, or through www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm.
Health care professionals and consumers should report adverse events related to the use of any medications, including suspected counterfeit medications to FDA's MedWatch Safety Information and Adverse Event Reporting Program:
Complete and submit the report online at MedWatch Online Voluntary Reporting Form, or
Download and complete the form, then submit it via fax at 1-800-FDA-0178.

Avoiding complications with Botox injections: 3 things to look for
Avoiding complications with Botox injections: 3 things to look for
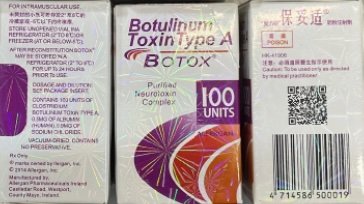
Counterfeit Version of Botox Found in Multiple States
Counterfeit Version of Botox Found in Multiple States
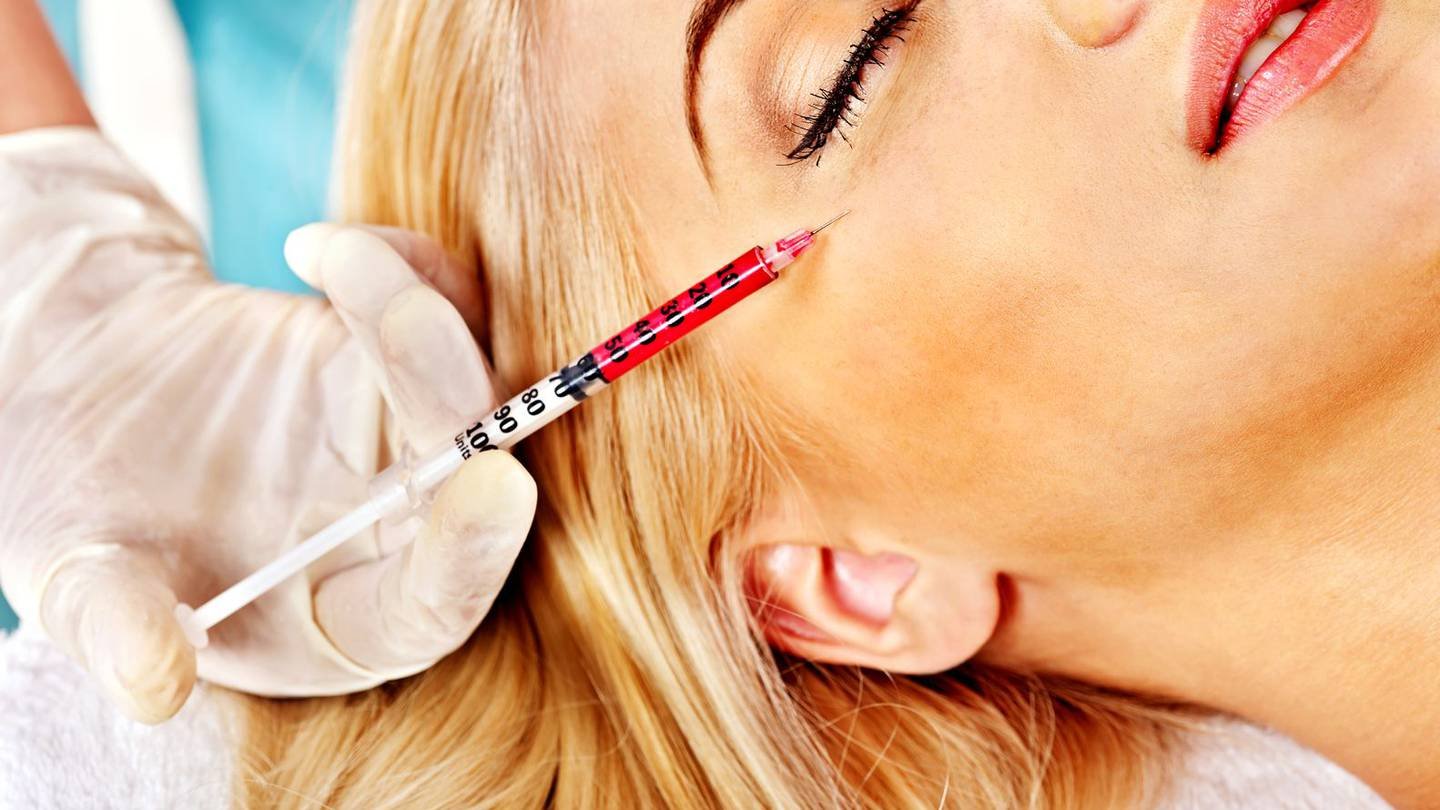
US health officials warn of counterfeit Botox injections
U.S. health officials issued a warning Tuesday about counterfeit Botox injections that have sickened 22 people.

Fake Botox Is Sickening Patients Across the U.S.
Fake Botox Is Sickening Patients Across the U.S.

Between Beauty and the Law: Analyzing Cosmetic Filler Litigations in South Korea for Safer Medical Practices
Between Beauty and the Law: Analyzing Cosmetic Filler Litigations in South Korea for Safer Medical Practices

FDA And CDC Launch Investigation Into Counterfeit Botox: What To Know
FDA And CDC Launch Investigation Into Counterfeit Botox: What To Know

Disastrous cerebral and ocular vascular complications after cosmetic facial filler injections: a retrospective case series study
Disastrous cerebral and ocular vascular complications after cosmetic facial filler injections: a retrospective case series study

Patients forced to seek emergency treatment over dermal fillers
Patients forced to seek emergency treatment over dermal fillers

Filler Gone Wrong: The Most Common Filler Mistakes Doctors See—and How to Avoid Having Them Happen to You
Filler Gone Wrong: The Most Common Filler Mistakes Doctors See—and How to Avoid Having Them Happen to You

Reversing Dermal Filler Blindness
Blindness is a rare but serious complication that can occur when a dermal filler is injected into a blood vessel, blocking the flow of blood to the eye, and causing vision loss.

Cosmetic patient sues doctor, nurse after Restylane® injection left her blind in one eye
Cosmetic patient sues doctor, nurse after Restylane® injection left her blind in one eye
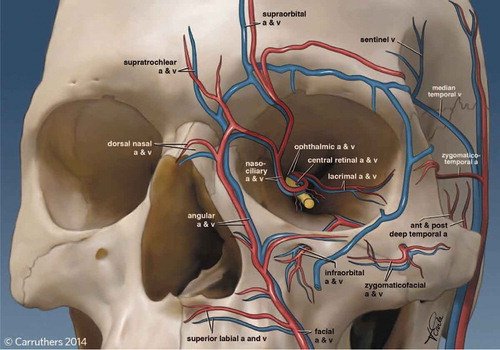
Cosmetic Filler–Induced Vascular Occlusion: A Rising Threat Presenting to Emergency Departments
Cosmetic Filler–Induced Vascular Occlusion: A Rising Threat Presenting to Emergency Departments

Lilly Ghalichi’s Filler Mishap Shows the Dangers of Vascular Occlusion
Lilly Ghalichi’s Filler Mishap Shows the Dangers of Vascular Occlusion

Can You Become Permanently Blind After Using Dermal Fillers?
Can You Become Permanently Blind After Using Dermal Fillers?
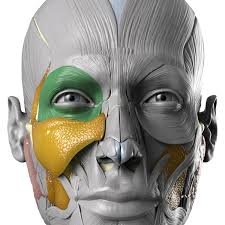
Vision Loss and Blindness Following Fillers
Vision Loss and Blindness Following Fillers

Popular cosmetic procedures can cause serious injuries including blindness
Popular cosmetic procedures can cause serious injuries including blindness

FDA Highlights Importance of Physician Training in Dermal Filler Injections
FDA Highlights Importance of Physician Training in Dermal Filler Injections

FDA Executive Summary General Issues Panel Meeting on Dermal Filler
FDA Executive Summary General Issues Panel Meeting on Dermal Fillers

Safety Update: Periocular Dermal Fillers
Safety Update: Periocular Dermal Fillers
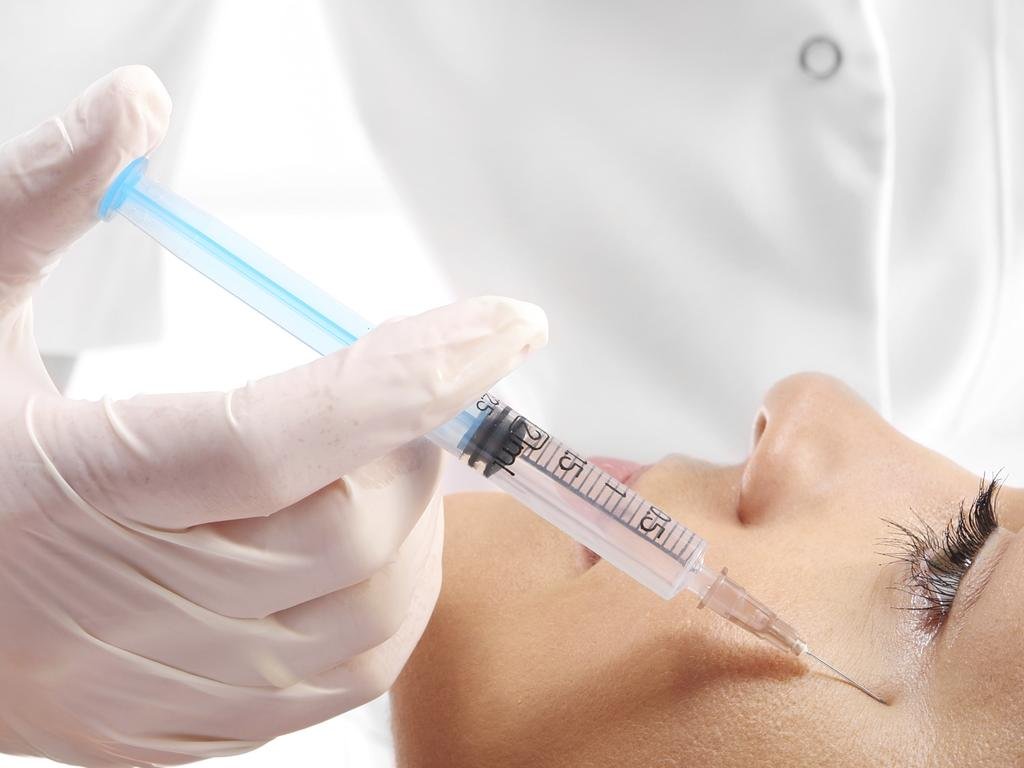
200 people report blindness after botched dermal filler procedures
200 people report blindness after botched dermal filler procedures